Date: 26 November 2013
Aspergillus terreus Thom. Conidial head of Aspergillus terreus. Conidial heads are compact, columnar and biseriate. Conidiophores are hyaline to slightly yellow and smooth walled.
Copyright:
With thanks to G Kaminski. D Ellis and R Hermanis Mycology Unit, Women’s & Children’s Hospital , Adelaide, South Australia 5006
Notes:
Colonies on CYA 40-50 mm diam, plane, low and velutinous, usually quite dense; mycelium white; conidial production heavy, brown (Dark Blonde to Camel, 5-6D4); reverse pale to dull brown or yellow brown. Colonies on MEA 40-60 mm diam, similar to those on CYA or less dense. Colonies on G25N 18-22 mm diam, plane or irregularly wrinkled, low and sparse; conidial production light, pale brown; brown soluble pigment sometimes produced; reverse brown. No growth at 5°C. Colonies at 37°C growing very rapidly, 50 mm or more diam, of similar appearance to those on CYA at 25°C.Conidiophores borne from surface hyphae, stipes 100-250 μm long, smooth walled; vesicles 15-20 μm diam, fertile over the upper hemisphere, with densely packed, short, narrow metulae and phialides, both 5-8 μm long; conidia spherical, very small, 1.8-2.5 μm diam, smooth walled, at maturity borne in long, well defined columns.Distinctive featuresVelutinous colonies formed at both 25°C and 37°C, uniformly brown, with no other colouration, and minute conidia borne in long columns make Aspergillus terreus a distinctive species.
Images library
-
Title
Legend
-
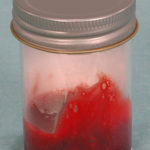
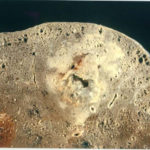
Pt MW Chronic Pulmonary Aspergillosis An example of bloody, thick, sputum from a patient with a destroyed left lung containing 2 large cavities, each with an aspergilloma.
 ,
, 
-
Further details
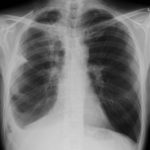
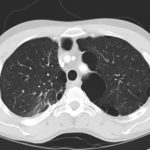
June 2009 – CT scan of the chest (two images D & E). These scans show residual large bullae, particularly notable in the apex of the left lung on both cuts. Small residual bullae remain in the right apex following a bullectomy which greatly reduced the size and number of these lesions.
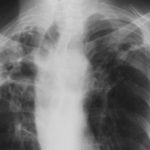
Pt RK Lung bullae caused by cannabis smoking complicated by pneumothorax and chronic cavitary pulmonary aspergillosis.
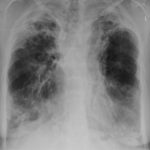
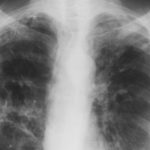
February 2009 – Chest x-ray showing bilateral apical bullae most marked on the right in association with a right pneumothorax. A chest drain is in-situ.
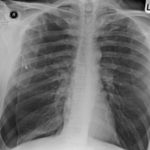
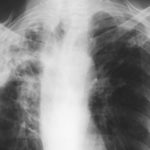
Late February 2009 – persistent apical bullae right upper zone with resolving pneumothorax. Chest drain still in-situ. Increasing right upper lobe shadowing possibly representing infection or haemorrhage.
April 2009 – Post-operative chest x-ray showing post apical bullectomy on the right, resolved pneumothorax but the interval development of fluid in the right costo-phrenic angle with air fluid levels consistent with recent surgery. Great reduction in apical bullae in the right apex but increasing consolidation proximally in the right upper zone and some fluid in the horizontal fissure. These findings are following an apical bullectomy and pleurodesis of his prior significant pneumothorax.
June 2009 – CT scan of the chest (two images – see above). These scans show residual large bullae, particularly notable in the apex of the left lung on both cuts. Small residual bullae remain in the right apex following a bullectomy which greatly reduced the size and number of these lesions.
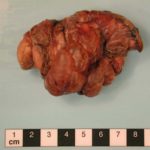
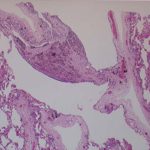
This patient underwent a bullectomy to remove part of the lung after developing lung bullae associated with cannabis smoking. The transverse section (G) of the specimen clearly shows the bullae along the left side and lower edge of the section. I and J demonstrate the dark pigment seen in accumulated macrophages. Tobacco smoking alone also causes a dark pigment, but evidence suggests that in marijuana smokers this pigment accumulates much faster and even with reduced exposure. The manner in which marijuana is smoked ie. without a filter and extended holding of the breath may contribute to this observation.
 ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
, 
-
History
Since March 2004, this 49 year old atopic lady has developed end-stage lung disease following a long history of severe steroid dependant asthma with recurrent infective exacerbations and colonisations by Aspergillus fumigatus and Pseudomonas aeruginosa. Both organisms had been isolated from sputum and previously an Aspergillus precipitins test was positive with an elevated total IgE level. Despite initial treatment with oral Itraconazole, chest X-rays were supportive of gross cystic bronchiectatic cavities in both apices and on CT scan a right upper lobe mycetoma (fungal ball). A diagnosis of allergic broncho-pulmonary Aspergillosis ABPA, with radiological evidence of bilateral apical fibrosis, was continuously treated with antifungal and Colomycin Nebulisers. This lady’s disease continued to deteriorate she developed Type 2 respiratory failure and was established on long term oxygen therapy (LTOT) of 2l/min via an oxygen concentrator at home and oxygen conserver when mobile. No recognised benefit was gained from a trial of non-invasive ventilation therapy and DEXA scan confirmed steroid induced osteoporosis resulting in a right sided rib fracture. Increasingly difficult venous access led to the insertion of a right sub-clavian Portocath for administration of intravenous antibiotic therapy. Systemic steroids, nebulisers and long term antifungal therapies did not prevent disease progression of ABPA to chronic cavitatory Aspergillosis.
She has been treated with voriconazole with some benefit, but slow deterioration, so that she is now 4l/min oxygen dependent 24 hours a day.
-
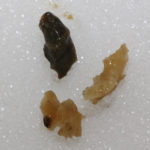
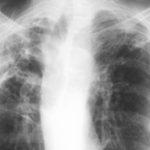
This man with chronic cavitary pulmonary aspergillosis, has been on continuous antifungal therapy for 4 years, initially itraconazole (with intracavity amphotericin B), then a short course of amphotericin B, then voriconazole with a period of interferon gamma, now posaconazole. He is co-infected with Mycobacterium xenopi, and has chronically abnormal liver function tests, of uncertain aetiology. Previously he coughed up plug-like material, and recently black hard material that is completely solid. This occurred following a 4 month period of sustained improvement on posaconazole (aspergillus precipitin titre has fallen from 1/32 to positive undiluted), but with relapsing M. xenopi infection
 ,
,  ,
, 






 ,
,  ,
,  ,
,  ,
,  ,
,  ,
,