ID 2: 389
Toxin: n
Trivial name:
Butanoic acid, 2-methyl-, 1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl]-1-naphthalenyl ester, [1S-[1α(R*),3α,7β,8β(2S*,4S*),8aβ]]-; (+)-Mevinolin; 6α-Methylcompactin; Altocor; Altoprev; Antibiotic MB 530B; L 154803; Lestric; Lostatin; Lovacard; Lovalip; Lovastatin; Lovastatin lactone; Lovex; MK 803; MSD 803; Mevacor; Mevinacor; Mevinolin; Mevlor; Monacolin K; Monacolin K lactone; Sivlor
Systematic name:
[3,7-dimethyl-8-[2-(6-oxotetrahydropyran-2-yl)ethyl]-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] 2-methylbutanoate
Molecular formulae:
C24H36O4
Molecular weight: 388.54
Chemical abstract number: 75330-75-5
Literature reference:
References URL:
Aspergillus Species known to produce this metabolite:
Structure image:
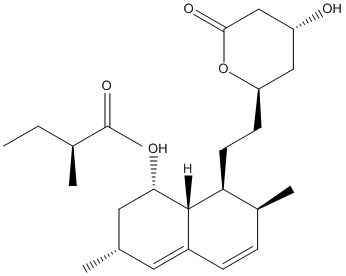
Date uploaded: 2008-07-07 16:58:01
Mycotoxin & Metabolites
-
Metabolite
Produced by (species)
Molecular weight
References
-
238.24
493.59
302.28
222.24
421.62
433.54
/species/clavatus, /species/a-terreus, /species/a-giganteus, /species/longivesica, /species/candidus, /species/a-amstelodami, /species/a-echinulatus, /species/a-fumigatus, /species/a-parasiticus, /species/a-repens, /species/a-variecolor, /species/a-versicolor, /species/a-carneus, /species/a-niveus, /species/a-pallidus
154.12
435.56
A1: 297.395 g/mol; A2: 297.395 g/mol; B1:279.38 g/mol; B2:279.38 g/mol; C1:295.379g/mol ;C2:295.379g/mol
/species/a-ochraceus, /species/a-quercinus, /species/a-sulphureus, /species/a-melleus, /species/a-ostianus, /species/a-sclerotiorum
170.16
Mycotoxin & Metabolite database
Aspergillus species produce a large number of secondary metabolites, sometimes referred to as extrolites. We attempt to list them all here and we also collect published papers.
Search Metabolite papers here
