ID 2: 80
Toxin: y
Trivial name:
10H-3,10a-Epidithiopyrazino[1,2-a]indole-1,4-dione, 2,3,5a,6-tetrahydro-6-hydroxy-3-(hydroxymethyl)-2-methyl-, [3R-(3α,5aβ,6β,10aα)]- (8CI); Gliotoxin (6CI,7CI); Aspergillin; S. N. 12870
Systematic name:
10H-3,10a-Epidithiopyrazino[1,2-a]indole-1,4-dione, 2,3,5a,6-tetrahydro-6-hydroxy-3-(hydroxymethyl)-2-methyl-, (3R,5aS,6S,10aR)-
Molecular formulae:
C13H14N2O4S2
Molecular weight: 326.39
Chemical abstract number: 67-99-2
Literature reference:
References URL:
Aspergillus Species known to produce this metabolite:
Toxicity:
Gliotoxin posseses a spectrum of biological activities including antibacterial and antiviral activities, and it is also a potent immunomodulating agent. Gliotoxin is also an inducer of apoptotic cell death in a number of cell types, and it has been found to be associated with some diseases attributed directly or indirectly to fungal infections. It is a secondary metabolite produced by a number of Aspergillus and Penicillium species.
It is a potent immunosuppressive metabolite and brings about apoptosis in cells. Because of its effects on the immune system it may have a place in transplant surgery. There is limited evidence for its occurrence in moulded cereals. A. fumigatus is a potent pathogen which can colonise the lungs and other body tissues after ingestion of spores. There is some limited evidence that gliotoxin may be formed in situ in such circumstances.
hamster LDLo oral 25mg/kg (25mg/kg) Veterinary and Human Toxicology. Vol. 32(Suppl), Pg. 63, 1990.
Link to PubMed
mouse LD50 intraperitoneal 32mg/kg (32mg/kg) Chemotherapia. Vol. 10, Pg. 12, 1965.
mouse LD50 intravenous 7800ug/kg (7.8mg/kg) Chemotherapia. Vol. 10, Pg. 12, 1965.
mouse LD50 oral 67mg/kg (67mg/kg) Chemotherapia. Vol. 10, Pg. 12, 1965.
mouse LD50 subcutaneous 25mg/kg (25mg/kg) Chemotherapia. Vol. 10, Pg. 12, 1965.
rabbit LDLo intravenous 45mg/kg (45mg/kg) VASCULAR: BP LOWERING NOT CHARACTERIZED IN AUTONOMIC SECTION. GASTROINTESTINAL: “HYPERMOTILITY, DIARRHEA” Journal of the American Chemical Society. Vol. 65, Pg. 2005, 1943.
rat LDLo intravenous 45mg/kg (45mg/kg) Veterinary and Human Toxicology. Vol. 32(Suppl), Pg. 63, 1990.
Link to PubMed
rat LDLo unreported 50mg/kg (50mg/kg) BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) Journal of the American Chemical Society. Vol. 65, Pg. 2005, 1943.
Structure image:
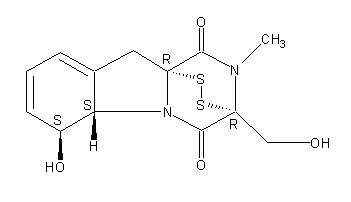
Date uploaded: 2007-09-25 13:12:16
Mycotoxin & Metabolites
-
Metabolite
Produced by (species)
Molecular weight
References
-
399.0
428.5
445.5
443.5
321.5
138.1
264.3
138.1
182.2
Mycotoxin & Metabolite database
Aspergillus species produce a large number of secondary metabolites, sometimes referred to as extrolites. We attempt to list them all here and we also collect published papers.
Search Metabolite papers here
