ID 2: 32
Toxin: y
Trivial name:
Spiro[furan-2(5H),9′-[9H]imidazo[1,2-a]indole]-3′,5(2’H)-dione, 4-[2-[1-(acetyloxy)-2-methylpropyl]-4-oxo-3(4H)-quinazolinyl]-1′,3,4,9’a-tetrahydro-1′-hydroxy-2′,2′-dimethyl-, [9’S-[9’α[4S*(R*)],9’aβ]]-; Fumitremorgin C; Tryptoquivaline; Tryptoquivaline A; Tryptoquivaline C
Systematic name:
9’R-(9’alpha(4S*(R*)),9’abeta))-4-(2-(1-(Acetyloxy)-2-methylpropyl)-4-oxo-3(4H)-quinazolinyl)-1′,3,4,9’a-tetrahydro-1′-hydroxy-2′,2′-dimethylspiro(furan-2(5H),9′-(9H)imidazo(1,2-a) indole)-3′,5(2’H)-dione
Molecular formulae:
C29H30N4O7
Molecular weight: 546.57
Chemical abstract number: 55387-45-6
Literature reference:
References URL:
Aspergillus Species known to produce this metabolite:
Structure image:
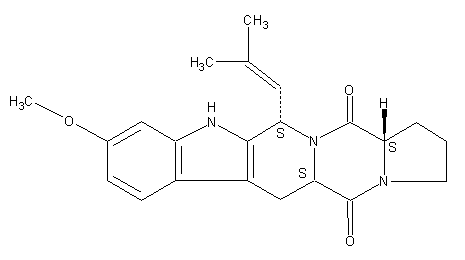
Date uploaded: 2005-11-29 00:00:00
Mycotoxin & Metabolites
-
Metabolite
Produced by (species)
Molecular weight
References
-
399.0
428.5
445.5
443.5
321.5
138.1
264.3
138.1
182.2
Mycotoxin & Metabolite database
Aspergillus species produce a large number of secondary metabolites, sometimes referred to as extrolites. We attempt to list them all here and we also collect published papers.
Search Metabolite papers here
