Invasive pulmonary aspergillosis (IPA) is an increasingly common opportunistic fungal infection usually occurring in patients with neutropenia and/or corticosteroid exposure. The lungs are involved in about 85% of cases of invasive aspergillosis. Factors that influence whether patients acquire this life-threatening infection include:
- Severity of neutropenia (especially <100 x106/L)
- Duration of neutropenia (especially >10 days)
- Neutrophil dysfunction (ie chronic granulomatous disease, SCID, late stage AIDS or chronic leukaemia)
- Corticosteroid dose and duration
- Other immunosuppressive agents such as methotrexate and anti-TNF inhibitors
- Inoculum (ie exposure to dust, compost or mouldy materials)
- Prior or current pulmonary disease (which probably increases risk)
- Genetic markers such as high IL-10 producers
- Severe systemic illness such as liver dysfunction and intensive care requirement
Evaluating patients for this infection requires a combination of clinical risk analysis and multiple modalities of diagnostic testing, including CT scanning of the thorax, fungal culture and microscopy of respiratory specimens, blood or bronchoalveolar fluid Aspergillus antigen testing, blood beta-D-glucan testing, biopsy of the lung (open, percutaneous or transbronchial) and other molecular testing of respiratory samples.
IPA is arbitrarily split into acute and sub-acute invasive pulmonary aspergillosis, on the basis of disease duration of less than one month or 1-3 months (Hope, 2005).
Indications for therapy
Survival from IPA requires early appropriate therapy (von Eiff, 1995; Caillot, 1997). This section makes recommendations for starting primary antifungal therapy based on clinical and diagnostic findings, separated by underlying condition.
In neutropenic patients or bone marrow or peripheral stem cell transplant recipients
Particularly high risk patients include those with persistent neutropenia (<500 x106/L) for at least 10 days or acute graft versus host disease (grade 2-4) or extensive chronic graft versus host disease on substantial immunosuppression. Any corticosteroid use increases risk. Triggers to start antifungal therapy include any new features such as
- cough
- persistent fever despite 5-7 days antibiotic therapy
- chest pain
- any shortness of breath
- any new radiological infiltrate (especially any nodular infiltrate, ‘halo’ sign, ‘air crescent’ sign or pleural-based lesion on CT scan).
- positive isolation of Aspergillus from any site
- hyphae demonstrated microscopically in bronchoalveolar lavage or a sterile site
- 1 very high (ie >2) or 2 positive sandwich ELISA tests positive for galactomannan in blood or BAL fluid
- positive PCR or other molecular diagnostic test for Aspergillus
- positive beta-D-glucan test in blood
In lung transplant patients
- isolation of Aspergillus from any site (further investigation will be necessary to confirm disease rather than colonisation.)
- Hyphae without yeasts demonstrated microscopically on bronchial or bronchoalveolar lavage, or bronchial biopsies
- positive PCR or other molecular diagnostic test for Aspergillus
- Persistent fever with new pulmonary infiltrates consistent with aspergillosis not responding to antibacterial therapy
- excess mucous material in airways, with no bacterial pathogen isolated and not responding to antibacterial agents
- positive sandwich ELISA tests positive for galactomannan BAL fluid
- positive serum Aspergillus antibody
In heart, renal liver and other solid transplant patients
- Isolation of Aspergillus from any site (further investigation will be necessary to confirm disease rather than colonisation.)
- Hyphae without yeasts demonstrated microscopically on bronchial or bronchoalveolar lavage, or bronchial biopsies
- positive PCR or other molecular diagnostic test for Aspergillus
- New cavitary or nodular lesions showing hyphae on percutaneous needle aspiration without a positive culture
- Persistent fever with new pulmonary infiltrates consistent with aspergillosis not responding to antibacterial therapy
- Positive sandwich ELISA tests positive for galactomannan BAL fluid
- Positive serum Aspergillus antibody
Patients with AIDS
- Isolation of Aspergillus from respiratory secretions together with new pulmonary infiltrates particularly cavitary or nodular lesions.
- New cavitary or nodular lesions showing hyphae on percutaneous needle aspiration or biopsy without a positive culture.
- New cavitary or nodular lesions with haemoptysis and negative samples on microscopy for acid fast bacilli (further diagnostic work necessary)
Patients on intensive care and patients with chronic obstructive pulmonary disease.
- Isolation of Aspergillus from respiratory secretions (needs further evaluation for colonisation or infection)
- New cavitary or nodular lesions or areas of consolidation showing hyphae on percutaneous needle aspiration or biopsy without a positive culture.
- positive sandwich ELISA tests positive for galactomannan BAL fluid
- Positive PCR or other molecular diagnostic test for Aspergillus on respiratory sample (needs further evaluation for colonisation or infection).
- Positive serum Aspergillus antibody.
Multiple tests positive, particularly characteristic CT findings with one or more positive microbiology tests positive is usually sufficient to confirm the diagnosis.
The EORTC/MSG consensus group have proposed diagnostic criteria for clinical and epidemiological study (Ascioglu, 2002), which have recently been updated (link to article in Diagnosis section). In clinical practice, patients should be treated before all criteria for possible or probable aspergillosis have been met, and if subsequently additional data are forthcoming that throw doubt on the diagnosis, treatment can be discontinued.
Medical therapy
The drug of choice for invasive aspergillosis is voriconazole (Herbrecht, 2002)(NOTE see Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America). Intravenous alternatives are amphotericin B, lipid-associated or liposomal amphotericin B, caspofungin or itraconazole. Oral alternatives are itraconazole and posaconazole.
Voriconazole should be used in all patients suspected of or with IPA initially unless:
- Patient has been receiving voriconazole prophylaxis (check plasma concentration)
- The patient is on or has recently been on interacting drugs which would prevent adequate concentrations being achieved (ie rifampicin, or other p450 inducers).
- There is severe liver dysfunction (reduce dose and measure plasma concentrations in those with moderate and mild hepatic dysfunction)
- There is moderate or severe renal dysfunction (for continued IV therapy only – concern about cyclodextrin carrier accumulation).
- The infecting isolate is resistant to voriconazole.
The optimal second-line choice is not known, but an amphotericin B preparation is preferred in neutropenia and an echinocandin (i.e. caspofungin) in non-neutropenic patients, depending on renal dysfunction and drug interaction considerations (rifampicin, cyclosporine and tacrolimus).
Consideration should be given to combination therapy with voriconazole and either caspofungin or micafungin in children, as the optimal voriconazole dose needs to be established in each child because of substantial variation in metabolism. Voriconazole dosing in children is generally higher than in adult patients, and starts at 7 mg/kg/dose q12hr for loading and maintenance dosing. The echinocandin may be continued until voriconazole plasma concentrations have been documented to be adequate.
Surgical resection
Surgery (lobectomy or wedge resection) is an important component of successful therapy in some patients. The indications for surgical resection of focal invasive pulmonary aspergillosis are:
- lesions impinging on the great vessels or major airways.
- significant haemoptysis
- persisting lung shadows prior to bone marrow transplantation or more aggressive chemotherapy
Many surgical resections have been done as an emergency, even if the patient remains neutropenic and thrombocytopenic (Albeda, et al, 1985; McWhinney, et al, 1993; Wong, et al, 1992; Robinson, et al, 1995; Caillot, et al, 1997; Habicht, et al, 1997). Platelet transfusions are given before and during the operation. Post-surgical survival has approached 80% with few surgical complications (Wong, et al, 1992; Massard, et al, 1993; Robinson, et al, 1995; Calliot, et al, 1997; Habicht et al, 1997, Pidhorecky, 2000; Yeghen, 2000; Gow, 2003; Matt, 2004; Cesaro, 2007).
Surgery has been deferred till hematologic recovery in other patients who were facing further chemotherapy or bone marrow transplantation (Lupinetti, et al, 1992; Young, et al, 1992,; Massard, et al, 1993; Moreau, et al, 1993; Marino, et al, 1994; Hoover, et al, 1997).
Prevention of subsequent recurrence of invasive aspergillosis has occurred in most (Young et al, 1992, Moreau et al, 1993, Massard et al, 1993; Nosari, 2007), but not all of these reports (Lupinetti et al, 1992).
Voriconazole
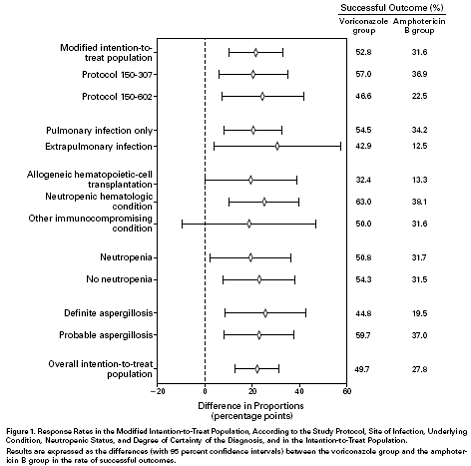
Voriconazole was studied at phase 2 in Europe in 116 patients and generated response rates in salvage and primary therapy of invasive aspergillosis of 48% and 52% respectively (Denning, 2002). The overall response rate for IPA was 60%. The dose used was 3 mg/Kg twice daily after 2 loading doses. This open uncontrolled study was followed a randomised study comparing conventional amphotericin B with oral voriconazole for 12 weeks unless a switch was made to another licensed therapy (Herbrecht, 2002). This showed that complete and partial responses occurred in 52.8% of those assigned voriconazole compared with 31.6% assigned amphotericin B, in 277 patients evaluated. The respective figures for IPA were 54.4% versus 34.2%. The confidence intervals did not overlap for almost all comparisons (see fig) indicating superiority of voriconazole. The same loading doses of voriconazole were used as in phase 2, but the IV voriconazole dose was 4mg/Kg twice daily, not 3mg/Kg twice daily.
There have been several additional studies and analyses published which show results in accord with these results (Patterson, 2005; Sambatakou, 2006; Walsh, 2002). In the original phase 2 study, it was observed that random plasma concentrations of voriconazole varied substantially. Although a small dataset, concentrations below ~250ng/mL were more often associated with failure and random concentrations higher than 10,000ng/mL more often associated with toxicity (Denning, 2002). The highly variable nature of metabolism with voriconazole has been confirmed in numerous other studies in adults and children (Purkins, 2002; Boyd, 2004; Walsh, 2004; Smith, 2006; Imhof, 2006; Mulanovich, 2007; Trifilio, 2007; Pascual, 2007; Pascual, 2008). Boyd et al associated some adverse events to high concentrations, ie confusion, collapse with low blood pressure, electrolyte disturbances, abnormal liver function tests, arrhythmia and hypoglycaemia. Smith et al, showed that the outcomes were worse if plasma concentrations fell below 2,000ng/mL. Most recently Pascual showed that trough concentrations blwow 1,00ng/mL were more often associated with failure, and concentrations above 5,500ng/mL with toxicity, especially neurological toxicity. Correction of low levels by dose escalation lead to responses and discontinuation of voriconazole in those with high concentrations lead to resolution of adverse events. All these data and analyses point towards routine monitoring of plasma concentrations of voriconazole, although the need is greatest in children, those of NE Asian origin (20% are slow metabolisers), those on potentially interacting drugs, those with poor response and those with any adverse effects.
Amphotericin B therapy
Since its introduction in 1959, (Kelmenson, 1959) intravenous amphotericin B deoxycholate (Fungizone) has been standard therapy for invasive pulmonary aspergillosis. Only in neutropenic paients has dose dependency been demonstrated; doses of 1-1.25mg/kg/d were more efficacious than lower doses (Denning & Stevens, 1990). In other patient groups, doses lower than 30mg/d ( 0.3-0.5mg/kg/d) are almost certainly less effective than doses of 0.5 mg/kg/d, but data are meagre and not entirely consistent (Denning & Stevens 1990).
Factors that favour the use of moderate to high initial doses of amphotericin B are; concentrations of amphotericin B are relatively low in lung tissue, most Aspergillus isolates are only moderately susceptible to amphotericin B and much animal model data indicates only modest outcomes for IPA (although good outcomes for disseminated aspergillosis. Thus the following recommendations for amphotericin B dosing are made:
- Aspergillus terreus is resistant to amphotericin B and alternative therapy should be used.
- In neutropenic patients, not receiving cyclosporin, use a starting dose of 1mg/kg/d amphotericin B (Fungizone) rising to 1.25 mg/kg/d if tolerated and disease progressive.
- In cyclosporin-treated patients, a lipid-based/liposomal amphotericin should be used, if possible. Check cyclosporin levels frequently.
- In patients with a degree of renal dysfunction (e.g. creatinine clearance <30 ml/min) or patients on other nephotoxic drugs such as gentamicin, a lipid-based/liposomal amphotericin B should be used.
- In patients with pre-existing chronic renal failure on dialysis, use 1-1.5 mg/kg/d conventional amphotericin B, if tolerated.
Lipid-associated and liposomal amphotericin B
Lipid-associated preparations of amphotericin B (Amphotec or Amphocil, Ablecet and AmBisome) are now widely used in the treatment of invasive pulmonary aspergillosis in the industrialised world. They are licensed for patients who have been intolerant to amphotericin B or whose infections are refractory to amphotericin B. None are superior to standard amphotericin B, but they are better tolerated, especially liposomal amphotericin B (Ambisome).
Amphotericin B colloidal dispersion (ABCD) (Amphocil or Amphotec) was evaluated in a phase I study for the treatment of invasive fungal infections after bone marrow transplantation (Bowden et al, 1996). The maximum dose of Amphocil achieved was 7.5 mg/kg. Of 14 evaluable patients with invasive pulmonary aspergillosis, only 5 failures of therapy occurred which is a remarkably good result in this patient group (Bowden, et al, 1996). In contrast, failure occurred in 15/23 (65%) patients treated with Amphocil (3-4 mg/kg) for invasive pulmonary aspergillosis in open label use (Oppenheim et al, 1995), which is more consistent with experience with amphotericin B. A large historically controlled study from 5 US, cancer or transplant centers compared clinical outcome and toxicity of Amphocil with historical controls treated with amphotericin B (White, et al, 1997). Response rates (48.8%) and survival rates (50%) among the Amphocil-treated patients were significantly higher than among amphotericin B-treated controls (23.4% and 28.4%, respectively) (White, et al, 1997). Renal dysfunction in evaluated patients (those who had received at least 7 days of either therapy) developed less frequently with Amphocil (8.2%) than with amphotericin (43.1%). Infusion related toxicities were not assessed (White, et al, 1997). A double-blind randomised study comparing Amphocil (6 mg/Kg/d) with amphotericin B (1-1.5mg/Kg/d) for invasive aspergillosis has been completed (Bowden, 2002). At end of treatment (usually up to 6 weeks), complete and partial responses for this ill and generally highly immunocompromised population from the USA were 18% for Amphocil and 23% for conventional amphotericin B in a total of 103 patients. An intent to treat analysis was similar in proportions – 13% versus 15%.
Amphotericin B lipid complex (ABLC) (Abelcet) and has been evaluated in open-label prospective salvage studies. Patients were enrolled who had failed amphotericin B or had developed nephrotoxicity (Walsh, et al, 1994; Hiemenz et al, 1995; Mehta et al,1997). Almost all patients were treated with 5 mg/kg. Of seven neutropenic patients treated for invasive pulmonary aspergillosis with Abelcet four had a complete response, one had a partial response and two were treatment failures (Mehta, et al, 1997). Reviews of over a hundred patients treated for invasive aspergillosis (predominantly pulmonary) with Abelcet in open-label emergency-use protocols, showed clinical responses (but not necessarily radiological response) in 40-60% of patients (Walsh et al, 1994; Hiemenz et al, 1995). In comparison, only 23% of historical controls treated with amphotericin B responded (Hiemenz et al.1995). Ablecet is less nephrotoxic than amphotericin B (Walsh et al, 1994; Hiemenz et al.1995). No randomised studies in IPA have been conducted with Abelcet.
As with Abelcet and Amphocil, data on the efficacy of liposomal amphotricin (AmBisome) in the treatment of invasive pulmonary aspergillosis has accrued gradually. Several retrospective case series have been published (Ringden et al,1991; Mills et al, 1994 Ng & Denning 1995;). Analysis of the three studies showed that 48% (31/64) of patients responded to therapy, many of these patients who had failed amphotericin B or developed nephotoxicity. Most patients in these 3 studies were treated with 5 mg/kg.
Two dose comparison studies of AmBisome in invasive aspergillosis have been conducted, one comparing 1 and 4mg/Kg, the other 3 and 10mg/Kg and one comparison with standard amphotericin B. The comparative study compared amphotericin B (1mg/kg) and AmBisome (5mg/kg) in neutropenic patients with probable or confirmed invasive aspergillosis (Leenders et al, 1997). Of 106 patients enrolled, 58 had proven or probable invasive aspergillosis. Neutropenic periods before and after randomisation did not differ. Patients were treated for a median of 23 and 24 days. There were 9 of 27 (33%) failures in the AmBisome group and 12 of 31 (39%) with a faster clinical resolution of disease in those treated with AmBisome, but equal time to radiological improvement. Thus AmBisome is clearly effective for invasive aspergillosis and possibly slightly superior to conventional amphotericin B.
Another randomised study, also in the neutropenic patient population showed that AmBisome 1 mg/kg/d and 4 mg/kg/d were essentially equivalent (study too small for a statistical definition of equivalence) with response rates of approximately 60% (Ellis et al, 1996). In the comparison of 3mg/Kg/d and 10 mg/Kg/d, almost all patients had neutropenia and the end of treatment responses were similar at ~50% with additional (nephro)toxicity at the higher dose (Cornely, 2007). As these patients were mostly neutropenic acute leukaemia patients, this 50% response rate compares with a response rate to voriconazole of 63%, although precise enrolment and evaluation parameters differ between the studies.
All these studies indicate that the response rates of conventional amphotericin B are similar to any of the lipid-associated amphotericin B products, with less nephrotoxicity and hypokalaemia with the lipid products. Voriconazole is superior to conventional amphotericin B and therefore probably the lipid products as well, although it has not been directly compared.
Amphotericin in Intralipid
Amphotericin diluted in lipid emulsion used in total parenteral nutrition (Intralipid) has been widely used in some centers as a relatively inexpensive alternative to other lipid preparations. Its use is not recommended as methods of preparation have not been standardized and dissociation of amphotericin B with the lipid mixture is likely leading to potentially toxic boluses of amphotericin B during the infusion.
Amphotericin B continuous infusion
Some centres use conventional amphotericin B reconstituted in glucose and infused over 24 hours. While there is a possibility of some light and temperature-induced minor degradation, in fact the clinical studies that have been conducted are generally supportive of this mode of delivery (Eriksson, 2001; Speich, 2002; Peleg & Woods, 2004; Hall, 2005; Maharom & Thamlikitkul, 2006). While reduced toxicity is likely with slower infusion times, outcomes are likely to be equivalent to conventional amphotericin B.
Caspofungin
Caspofungin was the first of a new class of antifungal drugs licensed in 2002 in humans, the echinocandins (Denning, 2003). The echinocandins are active against all common pathogenic species of Aspergillus, although never fungicidal. It was originally licensed based on its activity in invasive aspergillosis (Maertens, 2002). In this study patients unable to tolerate amphotericin B or failing amphotericin B or itraconazole were enrolled and treated with caspofungin monotherapy. Overall the response rate was 45% (complete and partial responses), in 87 patients, with wide variations in different patient groups. Those with proven IPA had a 38% and those with probable a 63% response rate. Overall, neutropenic patients fared worse (26% response rate), as did allogeneic HSCT patients (14%) whereas those enrolled for toxicity reasons did well (75%).
Numerous observational studies have been done following licensure (Glasmacher, 2006; Maertens, 2006; Antilla, 2007). Apparent response rates differ, probably because patients with different diagnostic criteria, underlying disease and severity of IPA were enrolled. In a recently presented prospective study of caspofungin as primary therapy for IA, the overall response rate was 33% (Viscoli, 2007). This lower response rate probably reflects more advanced IPA and underlying disease, as well uncertainty as to the efficacy of caspofungin in profoundly neutropenic patients. Pre-clinical work suggested extremely poor responses in profoundly and persistently neutropenic rabbits with IPA (Petraitiene, 2002), a finding in accord with the original salvage study in which a dismal response rate was recorded in those whose neutropenia did not resolve (Maertens, 2002). In contrast, caspofungin may be more effective in non-neutropenic patients.
Caspofungin has few drug interactions but ciclosporin use results in increased caspofungin concentrations whereas therapy with tacrolimus reduces tacrolimus concentrations. Rifampicin combination with caspofungin results in increased exposure to both compounds. Some additional low level interactions with phenytoin, dexamethasone, efavirenz, nevirapine, etc result in reduced caspofungin concentrations.
Micafungin
Micafungin was licensed in Japan in 2002 and its use there preceded all other echinocandins and all lipid-associated amphotericin B. After initial use in patients with subacute invasive aspergillosis (Kohno, 2004), a large study of micafungin in invasive aspergillosis has also been conducted (~290 patients) (Denning, 2006). In most patients, micafungin 75mg daily was added to existing therapy, if the patient was not responding to therapy. The dose of micafungin could be escalated to 150mg, then 225 then 300mg daily if the response was not thought to be adequate. All 331 cases were independently reviewed to determine diagnostic certainty and outcome, and inclusion of only those with confirmed invasive aspergillosis. Of these 225 were deemed to have IA and have received one dose of micafungin, an analysis set consistent with other studies. The overall response rate was 35.6%, with a 44% response rate in the 34 patients treated with micafungin alone. An initial dose of micafungin of 75mg daily is probably too low, and enrolment of patients who could also receive other therapy complicated the analysis. It is unclear if micafungin would be adequate therapy forA. terreus, as 0/10 patients in this study with infection with this species did not achieve a positive response.
Itraconazole
Concentrations of itraconazole in the lung exceed those in the plasma about two-fold (Grant et al, 1989). Itraconazole has been successfully used as monotherapy in the treatment of invasive pulmonary aspergillosis in many hundreds of patients following solid organ transplantation, bone marrow transplantation, in neutropenic and AIDS patients (de Buele, et al, 1988; Denning et al, 1989; Dupont, 1990; Denning, et al, 1990; Castelli et al, 1990; Denning, et al, 1992; Denning, et al, 1994; Kac, et al, 1995; Wallace, et al, 1996; Caillot, et al, 1997). Good responses in these studies occurred in more than 40% of patients. Many of the patients treated had failed amphotericin B previously. Most were evaluated using chest radiographs only.
In one study 6 of 7 (86%) profoundly neutropenic patients responded, despite neutropenia persisting for a median of 23 days (Denning et al, 1994). One randomized controlled trial has compared itraconazole with amphotericin deoxycholate (Van’t Wout et al, 1991) in the treatment of systemic fungal infections in neutropenic patients. The study was too small to discern any true difference between the regimens, no loading dose of itraconazole was given and the amphotericin B dose was relatively low.
Itraconazole has also been successfully used in subacutre and chronic invasive (necrotizing) pulmonary aspergillosis (Khoo & Denning, 1994; Saraceno, et al 1997). Response was seen in approximately 80% of patients treated with itraconazole in comparison to less than 40% of patients treated with intravenous amphotericin deoxycholate (Saraceno, et al ,1997). Responses in AIDS patients are not as good (Khoo & Denning, 1994).
In the treatment of invasive aspergillosis with itraconazole it is helpful to measure serum concentrations. A detectable serum concentration is necessary for response (Denning, et al, 1994) and in clinical experience a serum concentration of >5 mg/l (bioassay) or 1mg/l (HPLC) is predictive of response rates exceeding 50%. Itraconazole capsules are better absorbed with food (or an acid drink such as Coca Cola) and itraconazole suspension on an empty stomach. The bioavailability of itraconazole varies substantially from person to person, with some people having very low concentrations and others high concentrations. Interactions with rifampicin, carbamazepine, phenobarbitone, phenytoin and to a lesser extent rifabutin all reduce the serum concentrations of itraconazole to zero or nearly so.
Loading doses or initiation of IV therapy are also helpful in those with rapidly progressive disease. As the drug has a long (48 hour) half life, it takes about 14 days to reach steady state, using oral dosing. This delay can be reduced to about 5 days with loading doses of 200mg three times daily or intravenous therapy). Itraconazole has interactions with ciclosporin (reduce dose to 50% and monitor), tacrolimus (reduce dose to 30% and monitor), digoxin, warfarin (reduce dose to 50% and monitor), sulphonylureas, methylprednisolone, vincristine, cyclophosphamide, some inhaled corticosteroids, some antihistamines and HIV protease inhibitors. Itraconazole ahs a negative inotropic effect in some patients, which is potentially important in intensive care settings.
Posaconazole
Posaconazole is newly licensed in some countries for the salvage treatment of invasive aspergillosis. In vitro it is highly active against all common Aspergillus species (Oakley, 1997a;Diekema, 2003), and multiple other fungi. In animal models it is fungicidal, even during persistent neutropenia (Oakley, 1997b; Petraitiene, 2001). In an open label study, 106 patients over the age of 13 years were treated with 800mg daily of posaconazole, if they had failed or develop toxicity with any other medication (Walsh, 2007). In fact most had failed therapy (89%) with amphotericin B and/or itraconazole. Overall 42% responded (complete or partial) , with an additional 12% being unevaluable. In IPA, 31 of 79 (39%) responded. Higher response rates were seen with higher drug exposures; so the response rates were categorised by quartiles of average posaconazole concentrations:
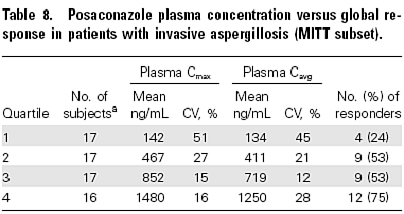
These concentrations are usually exceeded in patients, but there remains uncertainty about whether to monitor plasma concentrations, and dose escalation in volunteers was unsuccessful in increasing exposure, leaving clinicians with the only manoeuvre to increase food and oral fat intake with posaconazole if levels are found to be low. There are some important drug interactions with posaconazole including rifampicin and rifabutin (lower levels), cimetidine, tacrolimus, ciclosporin and phenytoin requiring monitoring. The drug should not be given with certain statins and drugs that may increase QT interval.
Combination therapy with amphotericin B
As outcomes of treatment of IPA have been poor and remain poor in certain patient groups and those with extensive disease, combination therapy is attractive.
Arguments in favour of combination therapy for IPA |
Commentary |
|
Synergistic combination |
No routinely synergistic combination of antifungals has been demonstrated in vitro, in vivo models of clinically. Most effects are additive or indifferent, therefore not a strong argument. |
|
Broader coverage of fungal pathogens |
Certainly true of the use of amphotericin B and extended spectrum azoles, but see below. If IPA is confirmed but the Aspergillus species is not known, then the addition of an echinocandin to amphotericin B would extend coverage to A. terreus, A. nidulans and A. ustus, which are generally amphotericin B resistant |
|
Different pharmacokinetic profiles, so better penetration into sanctuary sites. |
Possibly true, but never demonstrated to be clinically important, with possible exception of vitreous infection. Local amphotericin B used intra-vitreally in association with voriconazole systemically, for example. |
|
Early phase of IPA therapy in children with voriconazole requires another agent, until because of uncertainty about exposure and dose. |
Voriconazole is subject to rapid metabolism in children and establishing the correct dose for each child is difficult. Measuring plasma concentrations allows the dose to be individualised for each child, and the addition of an echinocandin during this phase f therapy provides cover. |
|
Reduction in emergence of resistance |
Theoretically possible, but never demonstrated and emergence of resistance on therapy of IPA has been rarely described. |
Arguments against combination therapy for IPA |
|
|
There is the possibility of antagonism |
Antagonism has been demonstrated in an unpublished study of terbinafine with conventional amphotericin B [Excess mortality rate in combination of ~25%] There is some in vitro and in vivo evidence of antagonism between azoles and amphotericin B and this recommendation is not recommended. Sequential therapy of amphotericin B followed by azoles such as itraconazole, voriconazole and posaconazole is probably not antagonistic (analysis of MSG itraconazole study (1994), voriconazole and posaconazole salvage studies), but azole pre-treatment or prophylaxis followed by amphotericin B may be problematic; data are scarce. |
|
Excess toxicity |
There is no evidence of additional toxicity or significant drug interactions with any antifungal combination except rifampicin and azoles. Flucytosine levels may accumulate with nephrotoxicity caused by amphotericin B. Any other additional toxicity is simply that intrinsic to each drug. |
|
Excess cost |
This may be problematic for the more costly drugs |
As the outcome with amphotericin B therapy is generally poor, combination therapy with amphotericin B is often considered. Slightly better results were observed with amphotericin B in combination with flucytosine (Denning & Stevens, 1990) but this could reflect bias in case reporting. In animal models, amphotericin B and flucytosine combinations were synergistic or indifferent (Carrizosa et al, 1975, Arroyo et al, 1977; Polak et al, 1982; Longman & Martin,1987; George et al, 1993;), but these experiments are difficult to interpret. In vitrocombinations were as likely to be synergistic as indifferent (Denning et al, 1992; Steinbach, 2003).
The only randomized controlled trial of amphotericin B alone versus amphotericin B with flucytosine in neutropenic patients with systemic mycoses had an 80% overall mortality, with no clear benefit for the combination. Amphotericin B doses were relatively low in this study (Verweij et al, 1994). Flucytosine thus is not recommended for routine use with amphotericin B in invasive pulmonary aspergillosis, but may be helpful in patients with cerebral, ocular or cardiac aspergillosis.
In vitro and animal data on the combination of amphotericin B and rifampicin (rifampicin) support their use together but is not recommended in patients because the use of rifampicin precludes the immediate subsequent use of an azole (reduced concentrations) (Arroyo et al, 1977; Denning et al, 1992; Stevens, 2000). Rifampicin has important interactions with all anti-Aspergillus azoles, dramatically reducing serum concentrations of these drugs (Patterson et al, 1996; Tucker et al, 1990; Denning et al, 1994) and preventing their subsequent use for at least 3 weeks after rifampicin is stopped.
There are numerous in vivo data related to amphotericin B echinocandin combinations for IPA (Luque, 2003; Clemons, 2005; Sionov, 2006; Dennis, 2006), but only a few clinical experiences. Perhaps the largest was the addition of micafungin to a failing regimen of amphotericin B (Denning, 2006). In this study those who received the combination has a slightly worse outcome than those who received monotherapy with amphotericin B. This result is mostly likely to reflect patient selection bias for combination therapy as physicians perceived that combination patients would do worse. However antagonism is possible, and the result is not supportive of the use of combination echinocandin and amphotericin B.
Azole (especially voriconazole) combinations with an echinocandin (especially caspofungin) are widely used. In children the combination allows time to ensure adequate voriconazole exposure and in adults is given in the hope that an additive or synergistic effect will follow. One specific study addressed 2 sequential cohorts of allogeneic HSCT patients treated only with voriconazole and then with the combination (Marr, 2004). Overall the combination resulted in a reduction of mortality from 68% at 90 days to 38% (p=0.008). This is suggestive of a benefit in poor prognosis patients, but needs further clinical evaluation for confirmation.
Specific recommendations
Voriconazole is the drug of choice for invasive aspergillosis. Individualisation of the dose improves outcome. Prior exposure to itraconazole or posaconazole does not preclude its use (different azole sub-class) but data are sparse. In extremely ill patients and in children, in whom the dose of voriconazole is uncertain, combination therapy with an echinocandin (caspofungin or micafungin) is appropriate. However, as in adult patients, optimal (higher) dosing of voriconazole in children is recommended over combination therapy. The best durg is optimized before additional agents are employed. Voriconazole plasma monitoring has the potential to improve outcome and reduce toxicity. Other drug interactions with voriconazole are substantial.
Amphotericin B is appropriate second-line therapy for patients requiring intravenous therapy or in whom the diagnosis is insecure and zygomycosis (mucormycosis) is still possible. It is preferred to an echinocandin in profoundly neutropenic patients over an echinocandin. The dose of AmBisome should be 3mg/Kg. The dose of Abelcet or Amphocil may be 5mg/Kg.
Caspofungin (70mg daily) or micafungin (150mg daily) may be used as second or third line therapy, or in combination with voriconazole, as stated above.
Posaconazole is highly active against Aspergillus and usually fungicidal. It is therefore a highly attractive choice, if oral therapy can be given, and when zygomycosis remains a possibility. With certain concomitant drugs it may be preferable to oral voriconazole to avoid certain drugs interactions, or in voriconazole intolerant patients.
Itraconazole may also be used to complete the treatment course in patients who cannot complete the course with amphotericin B if concurrent medication allows, or if continued therapy is deemed appropriate. It is not appropriate in first –line therapy of acute IPA, but may be valuable in less acutely ill patients. Resistance to itraconazole is rising. Plasma concentrations should be checked at least once.
David W. Denning FRCP FRCPath FIDSA FMedSci
Professor of Medicine and Medical Mycology
Director, National Aspergillosis Centre
Education and Research Centre
University Hospital of South Manchester (Wythenshawe Hospital)
Southmoor Road
Manchester M23 9LT UK
February 2008
References: