|
In a review of 228 cases of endobronchial fungal infection by Karnak et al.; ulcerative, pseudomembranous, obstructive and invasive tracheobronchitis were reported in 12 (10%), 9 (7%), 6 (5%) and 9 (7%) patients respectively. Parenchymal invasion was seen in tracheobronchial and pseudomembranous forms in 8 (7%) and 7 (6%) patients respectively. Interestingly, they also saw combination forms of tracheobronchial and allergic bronchopulmonary aspergillosis and obstructive bronchial aspergillosis and pneumonia in one case (Karnak, 2007).
Some authors suggest that rather than distinct entities, these morphologic variants may just represent different stages or manifestations in the development of Aspergillustracheobronchitis.
EPIDEMIOLOGY & RISK FACTORS
Overall, the incidence of aspergillus tracheobronchitis probably occurs in less than 7% of pulmonary aspergillosis cases (Kemper 1993). However in lung transplant recipients, frequencies of 20-35% in the first 6 months post transplantation have been reported (Husain 2004, Marjani 2008). Sadly, this infection was most often clinically unrecognized and almost 68% were identified on autopsy (Kemper 1993).
Patients who are at risk can be largely divided into two groups: lung transplant and non-lung transplant patients. Lung recipients are especially prone to Aspergillus infections owing to a number of factors: the ubiquitous nature of the organism, direct access of organisms to the allograft, the distorted native lung architecture that may harbor the organism (in the case of single lung recipients), and the more potent immunosuppression that lung allograft recipients generally require (Nathan 2000).
Aspergillus infection in lung transplants may manifest as endobronchial infection, particularly at the anastomotic site, as well as invasive and systemic disease (Kramer 1991, Westney 1996, Day 2006). In lung transplant recipients, tracheobronchitis is the most frequent type of infection occurring within three months after transplantation, whereas invasive pulmonary and systemic infections tend to occur later (Singh & Paterson 2005, Marjani 2008).
Isolation of Aspergillusspp. from respiratory specimens in this group of patients needs to be differentiated from saprophytic colonization with true infection. Asymptomatic colonization in the lung transplant recipient is reported to be between 10-85% (Horvath 1996, Alexander 2001, Perfect 2001). Attempts have been made to identify patients predisposed to infection after lung transplant, but such risks are poorly understood (Day 2006).
Pretransplant colonization with Aspergillus spp. was noted in 22-58% of cystic fibrosis patients and in 28% in non-cystic fibrosis patients undergoing transplantation. Of these cystic fibrosis patients who had pretransplant colonization, 25-42% subsequently developed tracheobronchitis within six months of transplantation (Nunley 1998, Helmi 2003). In one study, 34% of non-cystic fibrosis patients who had pretransplant colonization with Aspergillusdeveloped invasive aspergillosis (Helmi 2003).
In large published studies, reported that up to 46% of lung transplant recipients had colonization within six months of transplantation (Cahill 1997). They were eleven times more likely to develop invasive disease in the absence of prophylaxis (Nunley 1998).
Mehrad and colleagues reported in their lung transplant series, six of 133 patients (4%) were diagnosed for Aspergillus tracheobronchitis within the first six months after transplantation (Mehrad 2001). Almost all patients were asymptomatic and diagnoses were made during surveillance bronchoscopy. All lesions occurred in the transplanted lung and invariably involved the anastomosis line. The incidence was noted to be highest in the first year after transplantation (Horvath 1993, Cahill 1997, Mehrad 2001). This high incidence is probably because of their routine surveillance bronchoscopy, in which the disease is detected much earlier and patients are usually asymptomatic. Single lung transplant recipients developedAspergillus infections significantly later after transplantation than bilateral lung or heart-lung transplant recipients (median 4.9 months vs 2.1 months, p=0.019) (Westney 1996).
Although many authors have reported the progression from previously recognized Aspergilluscolonization to infection (Yeldandi 1995, Kanj 1996, Cahill 1997, Mehrad 2001, Singh & Husain 2003), the role of antifungal therapy for Aspergillus airway colonization in these patients is still remain unclear and unproven.
Associated underlying diseases and predisposing factors in non-lung transplant group include AIDS (Kemper 1993, Michels 1996), haematological malignancies (Kranke 2009), neoplastic disease, chronic obstructive airway disease (COPD)(Al-Alawi 2007), diabetes mellitus (Chang 2005, De Rosa 2009), organ transplant (e.g kidney transplant)(Kramer 1991, Mehrad 2001), systemic lupus erythematous (Angelotti 2002), post-influenza infection (Jariwala 1980, Boots 1999) as well as healthy individuals (Cha 2008, Sajal 2009).
Immunosuppressed patients with immunosuppressive drugs, chemotherapy & radiotherapy, prior and prolonged antibiotic treatment are also at risk. In addition, as reported by Young et al. Aspergillus tracheobronchitis cases were seen in patients with less neutropenia and less exposure to corticosteroids and anti-neoplastic agents. This probably contributes to the less invasive character of Aspergillus infection in these patients (Young 1970).
A pre-existing bronchiestasis may provide the stagnation and breach of surface necessary for the implantation of Aspergillus, which then acts as a source of infection. AlternativelyAspergillus may initiate blockage of a normal bronchus by mucus, with distal secondary infection which can be seen in obstructing tracheobronchitis cases (Hinson 1952).
AETIOLOGY
The aetiology and the reason for localization of aspergillosis to the tracheobronchial area without invasion of the pulmonary parenchyma as in most cases, remains unclear. Although initially thought to be confined to the tracheobronchial tree, there are some reports that suggest this disease may become invasive. Perhaps the combination of saprophytic colonization of the bronchial tree together with impaired mucociliary clearance, can lead to an opportunistic infections and subsequently causing a localization of the disease. This step may be facilitated by the host’s immunodeficiency such as using topical or systemic corticosteroids or altered local defence mechanisms such as prolonged presence of an endotracheal tube. As with more invasive forms of fungal infection, the degree of immunosuppression is probably the most important factor leading to bronchial wall invasion (Tasci 2000).
Aspergillus fumigatus is the most common species being isolated (74-84%), followed withAspergillus flavus (8-21%). Other Aspergillus species (3-5%) have also been isolated, such asAspergillus niger, terreus, nidulans and versicolor (Khoo & Denning 1994, Mehrad 2001, Wu 2009). It should be noted that Aspergillus terreus and Aspergillus nidulans are resistant to amphotericin B (Barnes 2006, Denning & Hope 2010).
CLINICAL MANIFESTATION
The clinical challenge in this disease is that both the early stages and progressive forms with extensive infection of the tracheobronchial tree and invasive infection usually develop without any apparent clinical symptoms or radiological changes. Patients can present with shortness of breath, dry/productive cough, fever, wheezing, chest pain or haemoptysis.
No specific physical signs are described, but wheezes and in particular a monophonic wheeze representing local airway obstruction may be found (Tait 1993, Sridhar 1995). Most patients are asymptomatic, especially in lung transplant patients in whom disease can be identified through bronchoscopic surveillance (Kramer 1991). Patients with obstructing bronchial aspergillosis usually become very breathless, prompting bronchoscopy, if they are not too hypoxic.
Death may occur as a result of direct intrathoracic invasion and subsequent dissemination, as well as fistula formation the airway and mediastinum, esophagus or pleura or from airway obstruction by the pseudomembrane formation.
RADIOLOGICAL AND THORACIC COMPUTED TOMOGRAPHY (CT) SCAN FINDINGS
Chest radiograph and CT scan is usually not helpful in acute tracheobronchitis since there are no remarkable findings (Franquet 2001). It is possibly because the infection is initially limited only to the tracheobronchial lining.
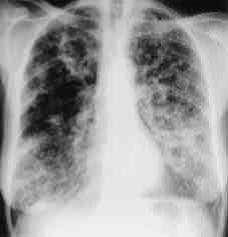
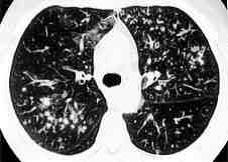
The radiological and CT findings of reported Aspergillus tracheobronchitis cases are usually non-specific and may mimic lung cancer, tuberculosis, amyloidosis, sarcoidosis or other fungal infections. It ranges from normal to patchy areas of consolidation or bronchopneumonia. Peribronchial consolidation, centrilobular nodules or branching linear-nodular opacities with a ‘tree-in-bud’ appearance, tracheal or bronchial wall thickening, proximal bronchiectasis with ground-glass attenuation may be either direct or indirect signs of tracheobronchial on a high-resolution CT (Figure 5 and 6)( Lortholary 1993, Logan 1994, Franquet 2001, Karnak 2007). These features correlated well with bronchoscopic and pathologic abnormalities, reflecting a progressive spectrum of the disease involving exclusively the large airways (Franquet 2001, Cha 2008). Obstructive tracheobronchitis can cause lobar or segmental lung collapse due to obstructive fungal casts (Karnak 2007).
|
| |
|
|
|
| Figure 5: Chest X-ray showing poorly defined bilateral nodular opacities (Al-Alawi 2007). |
Figure 6: High resolution CT showing centrilobular nodular opacities and branching linear opacities (tree-in-bud appearance) (Al-Alawi 2007). |
| |
|
ENDOSCOPY FINDINGS
Bronchoscopy with bronchial biopsy and culture is the diagnostic procedure suggested by many authors to make the diagnosis of Aspergillus tracheobronchitis (Kramer 1991, Kemper 1993, Denning 1996, Tasci 2006, Wu 2009). In ulcerative tracheobronchitis form, there is a focal fungal invasion ulcer of the tracheobronchial mucosa and/or cartilage (Berlinger 1989, Kramer 1991). The ulcers may become grossly blackened and necrotic; some may be coated with fibrinous exudates (Kemper 1993). In invasive tracheobronchitis, necrotizing white mucosa can be seen, sometimes discoloured green or black with local fungal spore formation (Biggs 1994, Sancho 1997, Boettcher 2000). These progresses in many cases to the pseudomembranous form which is characterized by extensive inflammation and invasion of the bronchial mucosa with a pseudomembrane composed of necrotic debris and hyphae. The pseudomembrane appeared as a mosslike covering the lower trachea, carina and major bronchi (Pervez 1985, Hines 1991, Higgins 1994). Obstructive tracheobronchitis, on the other hand, is described when thick mucus plugs loaded with Aspergillus are found in the airways with little mucosal inflammation or invasion (Denning 1991, Karnak 2007).
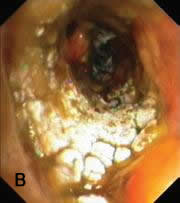
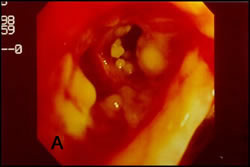
Only recently, Wu et al. has proposed a classification based on bronchoscopic morphology of the intraluminal lesions into four types (Table 1): superficial infiltration type (Type I), full-layer involvement type (Type II), occlusion type (Type III) and mixed type (Type IV). Wu added the mixed type which an overlapping bronchoscopic features seen in all morphological types (Figure 7) (Wu 2009).
Table 1: A proposed classification system of Aspergillus tracheobronchitis based on bronchoscopy findings.
|
CLASSIFICATION
|
BRONCHOSCOPIC FEATURES
|
|
Type I
Superficial infiltration type
|
Inflammatory infiltration, mucosa hyperaemia, mucosa oedematous and superficial ulcer which is confined to the mucosa and submucosa; mild plaques of pseudomembrane formation without obvious airway obstruction or deeper tissue invasion
|
|
Type II
Full-layer involvement type
|
Tracheobronchial lesions infiltrating through the matrix layer of bronchi, often with substantial and deep ulceration, extensive tissue necrosis with cartilage invasion and destruction of normal airway structures
|
|
Type III
Occlusion type
|
Airway obstruction or constriction > 50% of the original caliber of involved bronchi caused by extensive pseudomembrane formation, polypoid granulation or necrotic tissues as a result of Aspergillus infection, without definite proof of full-layer invasion
|
|
Type IV
Mixed type
|
Two or more different forms of typical bronchoscopic features coexisting at the time of diagnosis
|
CLASSIFICATION BRONCHOSCOPIC FEATURES
Type I
Superficial infiltration type
Inflammatory infiltration, mucosa hyperaemia, mucosa oedematous and superficial ulcer which is confined to the mucosa and submucosa; mild plaques of pseudomembrane formation without obvious airway obstruction or deeper tissue invasion
Type II
Full-layer involvement type
Tracheobronchial lesions infiltrating through the matrix layer of bronchi, often with substantial and deep ulceration, extensive tissue necrosis with cartilage invasion and destruction of normal airway structures
Type III
Occlusion type
Airway obstruction or constriction > 50% of the original caliber of involved bronchi caused by extensive pseudomembrane formation, polypoid granulation or necrotic tissues as a result ofAspergillus infection, without definite proof of full-layer invasion
Type IV
Mixed type
Two or more different forms of typical bronchoscopic features coexisting at the time of diagnosis
|
|
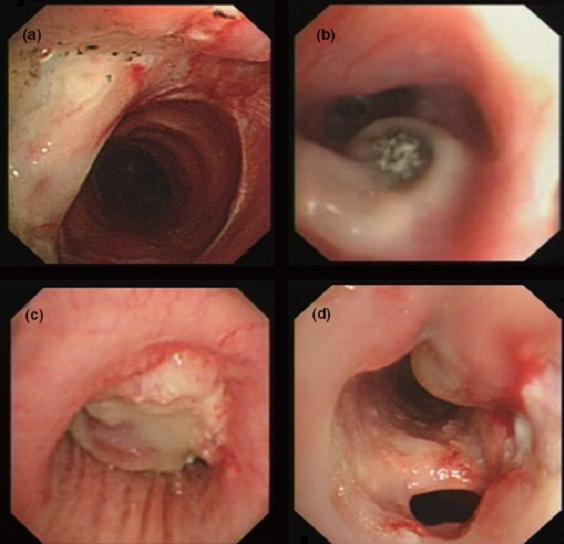 |
| Figure 7: Bronchoscopic manifestations of Aspergillus tracheobronchitis. (a) Type I. Inflammatory infiltration, mucosa hyperaemia and plaques of pseudomembrane formation in the lumen without obvious airway occlusion. (b) Type II. Deep ulceration of the bronchial wall. (c) Type III. Significant airway occlusion by thick mucous plugs full of Aspergillus without definite deeper tissue invasion. (d) Type IV. Extensive tissue necrosis and pseudomembrane formation in the lumen with airway structures and severe airway occlusion (Wu 2009). |
| |
|
There are some reports which suggest that the diagnosis can be facilitated by the use of endobronchial ultrasound (EBUS)-guided fine needle aspiration (Casal 2009) or positron emission tomography (PET) scanning in which, lesions caused by Aspergillus in the lungs and other organs are known to have increased fluorodeoxyglucose (FDG) activity on PET scanning (Wilkinson 2003, Sonet 2007).
LABORATORY DIAGNOSIS
CULTURE
A high degree of suspicion is required to make the diagnosis of Aspergillus tracheobronchitis. The gold standard of diagnosing Aspergillus infection is almost always confirmed by the isolation of Aspergillus spp. from the respiratory secretions or biopsies. However, the difficulty in isolating the fungus in antemortem specimens is sometimes frustrating although it is easily grown from environmental sources. In addition, dismal yield and poor specificity of isolatingAspergillus from blood cultures, contributes to late diagnosis.
The significance of Aspergillus isolated from sputum can be difficult to assess since positive sputum culture of Aspergillus occur in about 1-16% of healthy individual (Denning 1996).Aspergillus fumigatus colonizes readily within bronchi and damaged lung tissues and has pathogenic properties not possessed by other contaminating fungi (Clayton 1958). However, most authors believed that a positive sputum culture isolated from immunocompromised patients is always significant and warrants therapy (Nalesnik 1980, Yu 1986, Pursell 1992).
Aspergillus can be found in the bronchoalveolar lavage (BAL) or in bronchial washings by culture, by microscopy with detection of hyphae, by detection of the Aspergillus antigen, or by polymerase chain reaction (PCR). For identification of Aspergillus, BAL is cultured on Sabouraud dextrose agar, a fungal culture medium that is superior to the routine bacterial culture medium (Horvath 1996). Microscopy of BAL for Aspergillus hyphae is usually performed after haematoxylin-eosin or Grocott’s staining. Reichenberger and colleagues reviewed the value ofAspergillus culture and microscopy in BAL and found a sensitivity of 43% and a specificity of 100% in histologically proven cases of invasive pulmonary aspergillosis. The sensitivity for detecting invasive disease increases with multiple cultures (Horvath 1996). It has been reported that a positive fungal culture from secretion indicates a poor prognosis (Reichenberger 2002).
HISTOLOGICAL FINDINGS
Microscopy or histology of biopsies of pseudomembranes, mucous plugs or necrotic material help to established the diagnosis of Aspergillus tracheobronchitis in almost 94% of cases (Tasci 2006). However extra care need to be taken when taking deep biopsy specimens since cases of fatal haemorrhage have been reported and perforation into vessels or into the mediastinum may occur (Berlinger 1989).
Aspergillus spp. appears as slender septate hyphae that exhibit dichotomous branching in tissue section (Figure 8). Fungal-specific stains such as Gomori’s methenamine silver stain (GMS) and periodic acid-Schiff (PAS) should be applied in all cases suspected of invasive aspergillosis.
|
|
|
| Figure 8: Bronchoscopic biopsy demonstrated septate hyphae with branching at 45o (methenamine silver stain ×400). |
| |
|
Aspergillary bronchitis is a relatively indolent process which typically diagnosed only at autopsy (Berlinger 1989). It is characterized by superficial saprophytic growth of hyphae on the bronchial mucosa with superficial erosions and ulcerations with or without patchy membranes. It is present in 5 to 10% of patients with disseminated aspergillosis (Young 1970, Meyer 1973, Berlinger 1989). No criteria have yet been proposed for diagnosis although a recent study of six cystic fibrosis patients were characterized by repeat positive cultures, central bronchiectasis and clinical deterioration not attributable to another etiology (Shoseyov 2006). They also found that a slightly elevated serum IgE in one patient, a positive skin-prick test result to A. fumigatus in two patients, and weakly positive IgE-specific antibodies to A. fumigatus in one patient . All the patients did not respond to appropriate antibiotic treatment, but responded over varying periods of time and degree to antifungal medications (Shoseyov 2006).
In ulcerative tracheobronchitis, ulcers with shallow base and raised edges are present. Histologically there is evidence of necrosis and invasion of the abnormal area of bronchial mucosa and/or cartilage showing hyphae consistent with Aspergillus (Kemper 1993).
Pseudomembranous tracheobronchitis, on the other hand, is composed of the coagulation of fibrinosuppurative exudates, compact mass of Aspergillus hyphae, neutrophil infiltration, interstitial edema and vascular congestion. Extensive coagulation necrosis was seen in the underlying tissue. The tissues were infiltrated by inflammatory cells and the surrounding tissue was hyperaemic. This includes destruction of the underlying respiratory epithelium and transmural extension of the inflammation and hyphae to the level of the bronchial cartilage and peribronchial tissues, possibly reaching the lower respiratory tract, larynx and trachea (Pervez 1985, Berlinger 1989, Hines 1991, Ahn 2000). If these pseudomembranes dislodge, they can cause acute respiratory failure leading to airway obstruction (Zhang 2008). Where the tracheal epithelium was not affected by the Aspergillus growth, its cells were cuboidal or squamous metaplasia. These findings indicated antecedent irritation of the tracheal mucosa.
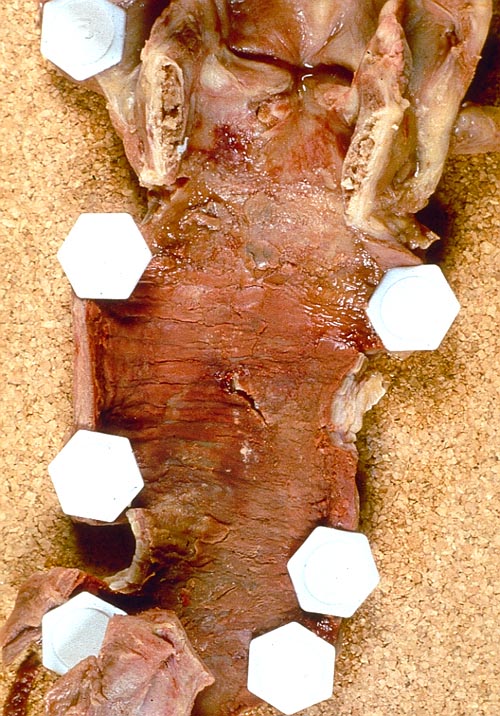
An example of pseudomembranous tracheobronchitis found on autopsy is shown in Figure 9.
|
 |
 |
 |
| Figure 9: Manifestations of pseudomembranous tracheobronchitis in a lung transplant patients on autopsy. A) Fibrinous or pseudomembranous bronchitis (arrow) with subocclusion of the airways (* indicates subocclusion of the airways by pseudomembranes); B) fibrinonecrotic material (arrow) from the airway shown in A, with subocclusion of the bronchial lumen (*); and C) Aspergillus hyphae (arrow) in the lumen without invasion of the necrotic bronchial wall (*) (Nicod 2001). |
| |
|
In obstructive tracheobronchitis, the mucous plugs which causing the obstruction is literally contains numerous hyphae of Aspergillus with minimal inflammation of the surrounding tissue. Orr et al. described presence of nodular lesions at autopsy in invasive aspergillosis cases as centrally necrotic gray-yellow areas surrounded by rim of haemorrhage (Orr 1978).
Interestingly, the presence of calcium oxalate crystals even in the absence of visualized conidia on pathologic examination has been associated with A. niger infection (Procop & Johnston 1997). This is due to the ability of A. niger produces oxalic acid with an affinity to bind tissue calcium and forming these crystals (Karnak 2007).
SEROLOGY
Galactomannan (GM) is a cell wall polysaccharide that is released by Aspergillus during hyphal growth. A commercially available sandwich enzyme immunoassay (ELISA) test (PlateliaTM Aspergillus, BioRad, France) measuring serum Aspergillus galactomannan antigen have become the mainstream and have replaced other conventional tests such as latex agglutination assays, because of their superior sensitivity (Kitasato 2009). Circulating GM can be detected at a median of 5-8 days before the clinical manifestations of invasive aspergillosis become apparent (Klont 2004). Some authors reported sensitivity as high as 81% and specificity of 86-95%, in various patients (Maertens 1999, Husain 2004). However, far lower sensitivity (30%) has been reported in lung transplant patients with invasive aspergillosis and none of the cases of tracheobronchitis was positive (Husain 2004). This can be explained that perhaps in early stages the infection is locally invasive and dissemination is unlikely. However, it may become positive later when the infection has become invasive and disseminated. Thus, the use of BAL may be more useful in this patient population.
Many investigators have reported the possibility of performing GM testing on BAL fluid samples from diverse patient population. BAL GM testing appears to be more sensitive (60-100%) and specific (82-100%) than serum GM in immunocompromised patients (Hsu 2010). This was also being reported by Meersseman and colleagues, examining the utility of BAL GM testing in critically ill patients. The test had a sensitivity of 88% and a specificity of 87%, a significant improvement over serum GM or BAL with culture and direct staining examination (Meersseman 2008). When BAL was used in lung transplant patients, the sensitivity was 60% and specificity was 95%, with positive and negative likelihood ratios of 14 and 0.41 respectively (Husain 2007). Few data are published on the utility of BAL GM in any forms of Aspergillustracheobronchitis. At present, the GM assays is not approved by the US Food and Drug Administration (FDA) for testing BAL, but it seems from the available data that such testing may improve the diagnostic yield of bronchoscopy with BAL in immunocompromised patients.
Polymerase chain reaction and nucleic acid sequence-based amplification are other advanced methods for establishing the diagnosis of aspergillosis tracheobronchitis, with few published data.
COMPLICATIONS
Complications may range from local complications to progression to the development of systemic aspergillosis. Local complication such as severe tracheobronchial obstruction is as a result of fungal masses and pseudomembranes, aggravating to acute life-threatening respiratory failures (Sayiner 1999, Tasci 2000). Fatal courses with death from bleeding occurred after transmural and peribronchial fungal fungal propagation, causing local necrosis, cartilage invasion or perforation into the mediastinum, and haemorrhage from bronchial-pulmonary fistula (Levy 1998, Boetcher 2000). In up to 40%, the infection was rapidly progressive causing respiratory failure and death (Kemper 1993).
TREATMENT
According to the IDSA guidelines, voriconazole is recommended (6 mg/kg IV every 12 hour for 1 day, followed by 4 mg/kg IV every 12 hour; oral dosage is 200 mg every 12 hour) as initial primary therapy in the treatment of Aspergillus tracheobronchitis (Walsh 2008). Voriconazole penetrates well into epithelial lining fluid (ELF) (Crandon 2009) with 7-fold higher concentrations than plasma in volunteers. Liposomal amphotericin B (3-5 mg/kg/day IV), amphoteric B lipid complex (5 mg/kg/day IV), caspofungin (70 mg day 1 IV and 50 mg/day IV thereafter), micafungin (IV 100-500 mg/day; dose not established), posaconazole (200 mg QID initially, then 400 mg BID po after stabilization of the disease) and itraconazole (dosage depends upon formulation) can be use as alternatives. The duration of the therapy is guided by clinical response which may extend from weeks to a year.
If amphotericin B is considered for use, liposomal amphotericin B is recommended in preference to deoxycholate amphotericin B because of its reduced nephrotoxicity and slightly better penetration into ELF compared into other amphotericin B preparations (Weiler 2009). Direct instillation of amphotericin B has also been used as an alternative approach in association with systemic therapy (Boettcher 2000, Hadjiliadis 2000). The use of nebulized deoxycholate or liposomal amphoterin B has been reported to provide some benefit for delivering high concentration of polyene therapy to the infected area especially in lung transplant patients, but is of uncertain value in therapy.
The guidelines do not recommend primary combination therapy based on lack clinical data. However addition of another agent or switch to another drug class for salvage therapy may be considered in individual patients.
In a large randomized controlled trial comparing voriconazole and amphotericin B for primary therapy of invasive aspergillosis, Herbrecht et al. reported the initial therapy with voriconazole led to better response (52.8% successful outcome), improved survival (70.8%) with fewer drug-related adverse events. Visual events was noted to be the commonest complaint, however it is usually transient and resolved without intervention (Herbrecht 2002). The same findings also being reported by Denning et al. in whom their three cases of Aspergillustracheobronchitis responded better with voriconazole than those with other sites of disease ( p< 0.001) (Denning 2002).
In a clinical study by Wu et al. involving nineteen cases of invasive Aspergillustracheobronchitis, seventeen were given oral or intravenous antifungal treatment whereas two were given nebulised treatment. Therapy includes itraconazole, liposomal and deoxycholate amphotericin B, voriconazole and caspofungin. Intermittent bronchoscopic intervention including electrocauterization, cryotherapy, mechanical debridement and intraluminal amphotericin instillation were performed as adjunctive treatments in most patients. Efficacious treatment was achieved in nearly 74% of the patients and mortality rate was 26.3%. This indicates a favourable outcome for patients with Aspergillus tracheobronchitis with early diagnosis and effective management (Wu 2009).
Tasci et al. reported pooled analysis of Aspergillus tracheobronchitis cases in which majority of the patients have underlying haemato-oncological disease. Twenty patients were treated with liposomal or conventional amphotericin B; intravenous or nebulised, voriconazole, caspofungin, itraconazole and flucytosine. They observed an 80% mortality rate and the rate was significantly higher in ventilated patients (94% vs 25%, p<0.05). However, they commented that it is difficult to distinguish whether the higher mortality rate is attributable toAspergillus tracheobronchitis or the underlying disease (Tasci 2006).
In addition, in Mehrad 2001 series, six lung transplant patients received oral itraconazole while two required additional surgical debridement. All patients responded to the therapy and none progressed to develop invasive pneumonia (Mehrad 2001).
Kemper et al. (Kemper, 1993) reported cases of HIV infection patients who developedAspergillus tracheobronchitis whom received combination therapy with amphotericin B and itraconazole. They observed that the disease can be rapidly progressive with almost 40% mortality in the affected patients.
BRONCHIAL TOILET
Repeated bronchoscopies for performing bronchial toilet were effective in removing the mucus plugs, pseudomembrane and relieving the patient’s symptoms (Angelucci 1991). Removal of the mycelial masses may be helpful because of the poor penetration of antifungals into the fungal masses, but the risk of bleeding complications seems to increase (Berlinger 1989). Thus extra care need to be taken in performing bronchial toilet because fatal bleeding during bronchoscopy has been reported in attempt to remove necrotizing pseudomembranes which has extended through the bronchial wall into a pulmonary vessels (Berlinger 1989).
ANTIFUNGAL PROPHYLAXIS
Prophylaxis for invasive aspergillosis is appealing in lung transplant recipients because of the high incidence of this infection in these patients; however, no formal recommendations can be made because of limited evidence. Current practice of antifungal prophylaxis is derived from clinical trials that have inadequate sample sizes, single-center noncomparable case series or case-control studies. The role of aerosolized amphotericin B still remains controversial, although some reported to be effective (Karnak 2007). Nebulised amphoterin B has been used both for prophylactic and therapeutic for Aspergillus lung disease including tracheobronchitis. It is generally well tolerated but may be associated with significant bronchospasm (Gryn 1993).
In a study involving 55 lung transplant patients, eighteen (33%) who were diagnosed withAspergillus infection used nebulized amphotericin B as fungal prophylaxis and those who developed tracheobronchitis forms usually have good prognosis (Montforte 2001). Nebulized liposomal or deoxycholate amphotericin B as fungal prophylaxis in lung transplant patients is preferred than itraconazole because of its safety with minimal systemic absorption and nephrotoxicity (Monforte 2009 & 2010). Monteforte et al. reported that the concentrations of nebulized liposomal amphotericin B remained high for 14 days, which is at adequate concentrations for prophylaxis of Aspergillus infection (Monteforte 2009). There is no significant systemic absorption of amphotericin B and no effect on respiratory function was observed. John Perfect has commented nebulized liposomal/deoxycholate amphotericin B may represent an alternate strategy for prophylaxis for high risk patients with neutropenia (Perfect 2008).
Mowat et al. showed that Aspergillus fumigatus can form coherent multicellular biofilm structures in vitro that are resistant to the effects of antifungal drugs (Mowat 2007). In addition Beauvais et al. have reported the presence of slimy extracellular hydrophobic matrix (ECM) of colony surface of Aspergillus fumigatus. The ECM is also seen between hyphae which glues together the hyphal threads of the network. Interestingly, Seidler et al, have demonstrated in vitro susceptibility testing of Aspergillus fumigatus biofilm on human bronchial epithelial and cystic fibrosis bronchial epithelial cells, so as to mimic the in vivo situation (Seidler 2008). They reported that all minimum inhibitory concentrations (MIC) were elevated in the presence of biofilm compared to the planktonic conditions. In the presence of biofilms the MICs for amphotecin B and liposomal amphotericin B ranged between 2 and >8 g/ml. For azoles (voriconazole, itraconazole and posaconazole), MICs increased from 0.125 and 0.25 g/ml to between 1 and 2 g/ml. In contrast, caspofungin and micafungin were ineffective in this study (MICs were >8 g/ml). Poor activity of the echinocandins is probably because echinocandins are fungistatic and active on growing hyphae. Thus it is likely that the drug cannot penetrate Aspergillus hyphae due to reduced permeability of the ECM and thus allowing Aspergillus to develop antifungal resistance (Seidler 2008). Future studies are needed to demonstrate in vivo growth of Aspergillus fumigatus biofilm especially in chronic lung diseases and its potential impact on infection and antifungal susceptibility.
OUTCOME
Severe obstructive and pseudomembranous tracheobronchitis are considered to be refractory to therapy and usually have a fatal outcome, whereas up to 82% of patients with ulcerative tracheobronchitis respond to antifungal therapy and/or surgical treatment. In twenty patients with pseudomembranous tracheobronchitis reported by Tasci et al., the overall mortality rate was 80%, and significantly higher in ventilated patients than in non-ventilated patients (Tasci 2006).
Overall, Aspergillus tracheobronchitis is fatal in about 23-48% of cases despite appropriate therapy with a markedly higher mortality in the pseudomembranous tracheobronchitis where mortality was well above 90% (Kemper 1993, Sayiner 1999, Singh & Husain 2003, Karnak 2007, Zhang 2008).
CONCLUSION
Aspergillus tracheobronchitis is now known as a discrete clinical entity or perhaps another disease manifestation of Aspergillus infection in the tracheobronchial area. Although various forms of clinical, bronchoscopy and histological findings have been reported, until date, full criteria for making each diagnosis within the overall disease entity have not been validated. Bronchoscopy is a critical to making diagnosis as well as providing specimens for microbiological and histological examination, and sometimes in providing bronchial toilet.
Sahlawati Mustakim,
Pathology Department,
Hospital Sungai Buloh,
Selangor Darul Ehsan,
Malaysia
dr.sahlawati@sel.moh.gov.my
|
|