Aspergillus bronchitis (AB) is a chronic superficial infection of the lower airways (trachea and bronchi) and part of the clinical spectrum of pulmonary aspergillosis, characteristically in non-immunocompromised patients. It may be caused by any of the pathogenic species of Aspergillus.
Patients with chronic pulmonary symptoms and evidence of Aspergillus in the airways that do not fulfill diagnostic criteria for chronic pulmonary aspergillosis (CPA), allergic bronchopulmonary aspergillosis or invasive aspergillosis may have AB. The prominent symptoms distinguish these patients from those with Aspergillus colonisation and therefore need specific treatment and follow-up.
Pathogenesis and definitions
Aspergillus bronchitis has been described in autopsy studies as a localized form of aspergillosis characterised by bronchial casts containing mucus and mycelia. The mycelia form small compact masses which are periodically expectorated. The fungus is not usually invasive in this form and the bronchial mucosa is usually preserved, however superficial erosion and progression to ulceration may occur (Young et al., 1970). Key histologic features of ‘non-allergic bronchial aspergillosis’ (Symmers, 1962) include definite but sparse tissue penetration, sometimes to lamina propria, chronically inflamed mucosa with thickening of the basement membrane (up to 20-fold) which appears as a broad hyaline membrane barely taking silver, fungal hyphal ends show vesicle-like expansion (5-10µm) in contact with or within thickened basement membrane, if the tissue is handled gently.
Most of the data on AB (including terms and definitions), derive from case series and reports. Invasive Aspergillus tracheobronchitis occurs primarily in patients with major immunocompromising factors particularly lung transplant recipients ( and occasionally only COPD, severe influenza or corticosteroid use for asthma), whereas AB is seen predominantly in those with bronchiectasis and cystic fibrosis. In order to avoid confusion with terminology, the term aspergillus bronchitis (AB) (Chrdle et al., 2012) is used for patients with chronic symptoms that do not have invasive disease, either tracheobronquial or disseminated. This is mainly attributed to different host characteristics since, in contrast to those with invasive aspergillosis, AB patients are not neutropenic or significantly immunosuppressed.
AB also differs from CPA as the lung parenchyma is usually normal but certainly without the usual features of cavitation, pleural fibrosis or nodules, nor are there prominent systemic symptoms. Furthermore, AB may occur in asthmatics but does not share the pattern of allergic response and sensitization as seen in ABPA or SAFS (Chrdle et al., 2012). Probably many patients with ABPA and consequent bronchiectasis also have AB, but it is not possible to be definitive, and so only the term ABPA is used.
Therefore, AB can be defined as a chronic bronchitis syndrome caused by Aspergillus in the airways, without important tissue invasion, lung parenchymal destruction or important allergic response.
Epidemiology
The burden of AB is not well known as diagnostic criteria have only been recently proposed. In the most recent case series published, among over 400 patients referred to the National Aspergillosis Center until 2011, only 17 were considered as having AB (Chrdle et al, 2012). From another case series, from 2002 to 2010 in 29 Spanish hospitals, 38 patients were considered to have AB (Barberán et al., 2014). However, this series included only cases with positive bronchoscopic examination. Other problems were the inclusion of patients with COPD with acute symptoms that needed hospitalisation who are predisposed to invasive disease.
AB also occurs in cystic fibrosis (Shoseyov et al., 2006). In a prospective study of 130 CF adults who produced sputum, 30% had AB (Baxter et al., 2013). Extrapolation of this figure by country and nearly globally, 11,314 patients with AB were estimated out of a near total adult CF population of 37,714 (Armstead et al., 2014) . It is not known if AB occurs in children with CF, although the first case was described at autopsy in a 3 year old (Wheaton et al., 1890).
Risk factors/Predisposing conditions
Only a few cases of AB have been published on the last few years and risk factors have not yet been clearly defined. In the largest case series, the mean age was 54 years and the majority of patients were female (82%) (unlike most other forms of aspergillosis in which males predominate), had bronchiectasis (86%), used inhaled corticosteroids (70%) and had mannose-binding lecitin (MBL) deficiency (56%) (Chrdle et al., 2012). Almost 25% had asthma and a one third had COPD.
It is not known what the predisposing factors for AB are in CF. The proportion of cystic fibrosis (CF) patients with A. fumigatus colonisation in respiratory secretions ranges from 6% to 58% (Armstead et al., 2014). Some of these patients will have AB, and distinguishing them probably requires detection of Aspergillus IgG in serum. Failure to respond to multiple antibiotics and a marked improvement with antifungal therapy further underscore the importance of the diagnosis of AB in CF patients.
Clinical features
The two following clinical presentations are the most common in AB patients:
- Chronic productive cough with tenacious mucus production, dyspnoea and difficult airway clearance.
- Recurrent exacerbations of preexisting airway disease with marked productive cough that have limited or no response to antibiotics.
Most, if not all, patients with AB have chronic productive cough. These patients frequently have large amounts of tenacious sputum, which may be any colour. Roughly half of AB patients complain of severe shortness of breath (MRC dyspnea score >4) and limited airway clearance has a major role. Occasionally patients may even need ventilatory support and therapeutic bronchoscopy for severe airway obstruction, so called ‘mucoid impaction’.
The second most common presentation is that of recurrent chest infections/exacerbations. As many patients have asthma, COPD and/or bronchiectasis their symptoms are often attributed to their preexisting lung condition. Bacterial infections are frequently held responsible for exacerbations and patients are usually treated with antibiotics. Patients with AB may have concurrent bacterial bronchitis. In patients with limited or short-lived response to antibacterial therapy, clinical suspicion of AB must be high. Oral corticosteroids may suppress symptoms.
Accordingly, AB should be included in the differential diagnosis of the chronic bronchitis syndrome (presence of cough and sputum production for at least 3 months in each of 2 consecutive years) as it may coexist with other lung diseases and even occur in patients with no underlying conditions.
Systemic symptoms are infrequent due to the lack of deep tissue invasion but extreme fatigue/malaise and significant weight loss have been reported. In like manner, hemoptysis is very rare. The clinical manifestations of AB are summarized in table 1.
Table 1. Clinical manifestations of Aspergillus bronchitis (decreasing order of frequency) |
|
|
|
Diagnosis
As aforementioned, diagnosis requires high suspicion and a thorough workup of other Aspergillus-related conditions. Diagnostic criteria have been proposed by Chrdle et al. in 2012 (Table 2). These criteria also apply to adult patients with CF, as described by Baxter et al. in 2013, although the presence of Aspergillus IgG in serum above 75 mg/L is probably seen more commonly in CF than other patients with AB.
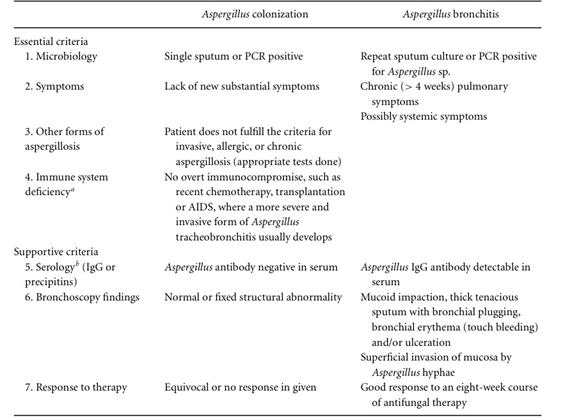
Some patients with cough have an elevated Aspergillus IgG. These patients should be evaluated for AB seeking Aspergillus in the airways by culture, PCR and/or bronchoscopy. Gastroesophageal reflux disease (GORD) and vocal cord dysfunction should also be considered, especially if the patient is hoarse, with gastroscopy, pH manometry, video swallow and/or direct laryngoscopy.
Microbiology
Isolation of Aspergillus species and/or positive PCR in the respiratory tract of patients with different lung conditions are common and might represent colonization only. Repeat isolation or positive PCR are therefore necessary for the diagnosis of AB. While A. fumigatus is the most common species implicated, A. niger, A. terreus (Chrdle et al., 2012), and A. flavus (Rummens et al., 2014) are described as causative agents of AB.
Sputum samples are often the preferred samples as they are non-invasive and can be obtained on each office visit or even collected at home and submitted through the mail. The sensitivity of sputum cultures varies greatly between reports and this may be related to a lack of standardisation or limitations of current standard methods. Sample volume appears to have an important role on sputum culture sensitivity, as shown by Fraczek et. al in 2013. In their study, high volume sputum cultures had a significantly higher fungal recovery rate, up to ten times higher than the local standardized method. These findings are similar to a previous study in 2012 with sputum from COPD patients, that showed that not only the quantity but also the media used play a role in fungal isolation (Pashley et al., 2012).
Sputum PCR is more sensitive than culture (Fraczek et al., 2013), and patients with 2 or more strongly positive Aspergillus PCR samples can be defined as having AB, with a compatible clinical picture, in the absence of culture.
Bronchoalveolar lavage may yield Aspergillus or Aspergillus antigen and/or positive PCR for Aspergillus but there are no comparative studies showing superiority of either method in the context of AB. A strongly positive galactomannan or PCR signal from bronchoalveolar samples is also helpful in contributing towards a diagnosis of AB.
Ruling out CPA and ABPA/SAFS
CPA can be ruled out based on chest radiology. Patients with Aspergillus nodules may have minimal parenchymal disease, a postive sputum culture for Aspergillus and detectable Aspergillus IgG antibody (Kosmidis et al., 2015). Progressively worsening lung imaging and function not attributed to underlying lung disease does not occur in AB – airways obstruction is common and problematic when mucus plugging is maximal. ABPA is associated with Aspergillus sensitization defined by a positive skin test or specific IgE combined with a high total IgE levels (in ABPA > 1000). Sensitisation may be seen in AB, but the other characteristic features of ABPA are not seen in AB, with the exception of a productive cough. Table 2 summarizes the different characteristics among Aspergillus-related diseases:
Table 2. Succinct comparison between AB and other Aspergillus-related conditions
|
Characteristics |
Invasive aspergillosis |
ABPA |
CPA |
AB |
|
Host factors |
Significant immunossupression, neutropenia, transplantation |
Asthma or CF and other allergies |
Chronic pulmonary disease (Asthma, COPD, bronchiectasis) , lung sequelae (TB, sarcoidosis, surgery, radiation) |
Chronic airway disease (Bronchiectasis, asthma, COPD,) |
|
Onset |
Acute |
Chronic |
Chronic |
Subacute or chronic |
|
Systemic symptoms |
Common |
Uncommon |
Common |
Uncommon |
|
Respiratory symptoms |
Mild and nonspecific |
Productive cough with mucus plugs, wheezing, dyspnea |
Productive cough, hemoptysis, dyspnea |
Productive cough, recurrent chest infections, dyspnea, thick sputum/plugs |
|
Chest imaging |
Halo and air crescent signs, consolidation, pleural-based sharply angled consolidation/ infarction |
Airway obstruction with mucus, central bronchiectasis |
Progressive cavity formation, fibrosis, lung volume loss, aspergillomas, nodule(s). |
Bronchiectasis or normal or attributable to underlying lung disease |
|
Aspergillus IgG and precipitins |
Usually negative, may seroconvert |
Positive or negative |
Positive |
Positive or negative |
|
Serum IgE |
Occasionally positive |
Always elevated, >1000 in ABPA |
Normal or mildly elevated (70%) |
Normal or mildly elevated |
|
Fungal-specific IgE or skin prick tests |
No data |
Positive |
Often low level positive |
Negative |
Serology
Aspergillus-specific IgG may be positive but patients with negative results are often seen (unpublished data). This might occur due to insufficient sensitivity or lack of a disease-specific cutoff. In CF patients, a diagnostic cut-off of 75mg/L was established for the ImmunoCap assay (Baxter et al., 2013); no other assays have been studied in this context.
Bronchoscopy
Bronchoscopy may be normal or reveal hyperaemic inflamed mucosa that bleeds easily, or features that correspond to those classified as “type I: superficial infiltration type” by Wu et al. in 2010. These findings include:
- inflammatory infiltration
- mucosal hyperaemia and/or hydropsia
- superficial ulcer(s) confined to the mucosa and submucosa
- mild plaques of pseudomembrane formation without obvious airway obstruction or deeper tissue invasion
The presence of sticky secretions, hyperaemic mucosa and whitish plaques by bronchoscopy has been shown to be a quick and reliable way to diagnose fungal bronchitis (Yazıcıoğlu et al., 2013).
Some patients have airway obstruction due to tenacious mucus (Chrdle et al., 2012). In such cases, bronchoscopy may be both diagnostic and therapeutic. Bronchoscopy is also very useful for obtaining samples for microbiology. Bronchoscopy is however invasive and requires sedation.
Treatment
Azoles are the cornerstone of Aspergillus bronchitis therapy. The use of both itraconazole and voriconazole has been previously reported with good outcomes in non-CF patients. Many patients get dramatic benefit with near resolution of respiratory symptoms and reduction or even cessation of chest infections. No comparative randomized trials have been done. A typical treatment duration is 4 months. Relapse on discontinuation of therapy is common (Chrdle et al., 2012). Such patients usually respond to retreatment. It is not clear if CF patients should be treated or not or how best to evaluate response. Itraconazole interacts with some inhaled steroids, and the dose of inhaled steroid should be minimised, or even stopped if not indicated (ie for asthma).
Some patients have contraindications to azole therapy and others develop adverse effects or acquire an azole-resistant isolate of Aspergillus (Howard et al., 2010). In such cases, according to sensitivity testing, a different azole or inhaled amphotericin B may be alternatives. Some patients also seem to improve with azithromycin. Immunisation with pneumococcal and/or haemophilus vaccine may prevent bacterial bronchitis and some exacerbations. These features may be related to benefits on the underlying airway disease.
Follow-up
Response to therapy should be periodically evaluated, taking into account clinical, laboratory and bronchoscopy findings. Azole drug levels should be monitored early during therapy and periodically if therapy is prolonged or symptoms recur on therapy (Hope et al., 2008). Aspergillus PCR signal strength can be used to check that antifungal therapy is adequate. If a strong PCR signal is present despite ‘therapeutic levels’ of azole, antifungal resistance should be suspected. Multiple repeat sputum cultures and/or bronchoscopy may be warranted to establish this.
In patients with bronchiectasis, it is not known if AB contributes to airway damage and progression of bronchiectasis. In CF patients, there was more FEV1 loss over 2 years compared with uninfected patients, but slightly less than those who were sensitized or had ABPA (Baxter et al., 2013).
João A. G. G. Prats MD
Medical Mycology Group
Federal University of São Paulo (UNIFESP)
São Paulo
Brazil
David W. Denning FRCP
National Aspergillosis Centre
University Hospital of South Manchester
The University of Manchester.
References:
Case histories:
