Submitted by GAtherton on 20 July 2016
We discussed several weeks ago the advantages to drug companies as well as doctors and their patients of using an existing drug – one that has already passed clinical trials for safety (Phases 0 & 1) and largely now only needs to be tested for efficacy (Phase 2 & 3). This reduces the time needed for drug development and testing quite markedly.
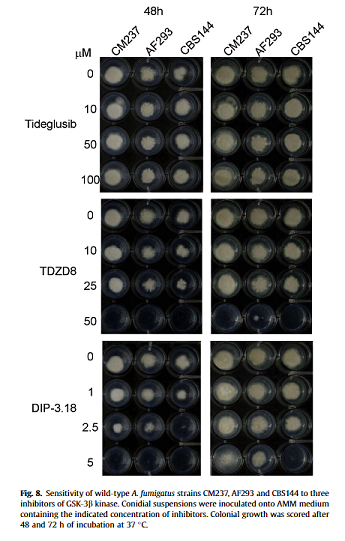 At that time we discussed an antifungal drug that was being repurposed to treat cancer, but this recent paper describes a drug that is showing promise for the treatment of aspergillosis. Glycogen synthase kinase (GSK) function is well characterised in humans and is active in several metabolic pathways including e.g. glucose regulation, consequently inhibitors of GSK (of which there are many) have been tested for the treatment of diabetes, bipolar disorder, Alzheimers disease and some types of cancer.
At that time we discussed an antifungal drug that was being repurposed to treat cancer, but this recent paper describes a drug that is showing promise for the treatment of aspergillosis. Glycogen synthase kinase (GSK) function is well characterised in humans and is active in several metabolic pathways including e.g. glucose regulation, consequently inhibitors of GSK (of which there are many) have been tested for the treatment of diabetes, bipolar disorder, Alzheimers disease and some types of cancer.
Following the discovery of GSK in Aspergillus fumigatus Sebastian et.al. have extended this list of GSK inhibitor applications to include treatment of aspergillosis. Tests in vitro show that several GSK inhibitors are able to completely reduce the growth of A. fumigatus (see figure), suggesting that they may be effective in reducing the growth of Aspergillus infections in vivo.
The authors also suggest that as we know that GSK inhibitors may also increase the effectiveness of the host’s immune system there may be a synergistic interaction between the activities mentioned which will work to increase the benefit of the drug for infected patients – though this claim has yet to be tested.
The arrival of this new drug as an antifungal medication would also be welcomed by doctors as it would be a brand new class of antifungal drug towards which little initial resistance is likely to be found. This would be an improvement on for example using one of the several available antifungal drugs from the triazole class of drugs when an infecting fungus is already resistant to an azole drug. In those circumstances the fungus is more likely to already be resistant to the new drug or to become resistant shortly afterwards.
It could well be that clinical trials of the effective GSA inhibitors can begin relatively soon – there is no information in the paper that indicates whether the dose of GSK inhibitor used in this experiment is equivalent to the doses already tested in vivo. If not there may need to be initial studies in test animals to check drug toxicity prior to testing in human patients.
News archives
-
Title
Date


