Submitted by Aspergillus Administrator on 29 November 2010
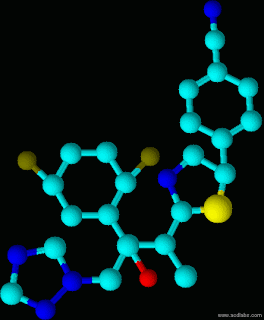
Isavuconazole is a novel, water-soluble, broad spectrum antifungal medicine developed by Basilea. It is available in an intravenous form or orally – with high bio-availability, as a capsule. It is currently undergoing multiple clinical trials – for yeast infections, aspergillus infections, aspergillosis in renally impaired patients and for rarer mould infections.
The pharmaceutical companies Astellas and Basilea have a co-development and co-promotion agreement for a worldwide license – agreed earlier this year.
Pre-clinical and clinical data so far indicated that isavuconazole has the potential to overcome many limitations of current therapies for invasive fungal infections. It has predictable and dose proportional pharmacokinetics – which is important to maintain therapeutic drug levels in patients.
Importantly the IV- dose form has a good potential to be given safely to patients with kidney impairment.
The phase III clinical trial for the treatment of invasive aspergillus infections will be continued as recommended by the Data Safety Monitoring Board based on results from the first 180 patients. Basilea and Astellas have announced a change in the primary endpoint for monitoring the phase III trial (more info here) which will mean that the trial will need to show that isavuconazole is actually saving lives instead of just improving clinical symptoms- when compared to first line treatment – in this case voriconazole. Basilea states that this change will not impact on the trial’s timeline, which should be complete by early 2011 with results due in 2013. Isavuconazole has been fast tracked in the US by the FDA.
News archives
Showing 10 posts of 953 posts found.
-
Title
Date